Healthcare & Medical App Development
The Definitive Guide (2021)
Medical app development focuses on developing mobile apps for use by patients, carers and healthcare professionals (HCPs). They’re also referred to as mHealth applications and are developed to help patients manage a particular medical condition and communicate with clinicians. HCPs use them to diagnose patients, perform medical calculations and access electronic health records.
Last update: January 2021
In this expert-written guide you’ll learn everything you need to know about developing a health app.
In Part 1 we’ll take you through the market opportunities and talk about how medical apps can help to solve some of the healthcare challenges we currently face and how the Internet of Things (IoT) is shaping the future of healthcare.
In Part 2 we’ll provide you with some practical advice on developing medical apps. From developing your mobile health (mHealth) strategy, to choosing the right app developer and factoring in medical compliance.
So, if you’re looking to develop a highly successful mobile app for use by either patients and/or healthcare professionals then this guide will provide you with a blueprint for success. It has been compiled by two individuals that have over 5 years hands on experience in developing health apps including apps that are classed as medical device software (MDSW) and have been CE marked.
Let’s make a start…
Don’t have time to read the whole guide right now?
No worries. Let us send you a copy so you can read it when it’s convenient for you. Just fill in the form and we’ll send you a PDF (takes 5 seconds):
[hubspot type=form portal=8087817 id=ccd53c5c-5bd0-4c34-b2c7-30eec43841bb]
With approximately five billion smartphone users worldwide, there are huge opportunities for mobile medical apps and other mobile health (mHealth) technology to revolutionise healthcare and patient outcomes and experience. This is reflected in economic forecasts and a report by the market research firm Mordor Intelligence suggests that the global mHealth market will reach $62.84 billion in 2021. Among the key drivers of this rapid growth are ever-increasing smartphone penetration rates.
According to Deloitte’s Global Mobile Consumer Survey (GMCS), 88% of the UK population either own or have access to a smartphone. Sporting the highest ownership rate of 94%, are the 25 to 34 year olds. The 35 to 54 year olds and the over-55 age group follow with adoption rates of 91% and 80%, respectively.
An interesting finding of the GMCS survey is that smartphones have increased quite rapidly over a relatively short time. In 2012, only about 50% of UK adults owned one. And, although the year-on-year growth appears to be gradually slowing down, smartphones are, and are expected to remain, the electronic device with the highest penetration rate.
There is evidence to suggest that smartphones are the most successful electronic devices also among healthcare professionals. In 2015, a team from Imperial College, London, conducted a study of over 6,000 hospital doctors and nurses from five NHS hospitals.

The results, published in the British Medical Journal, show that 73.5% of surveyed doctors and 64.7% of surveyed nurses owned a tablet, but 98.9 and 95.1%, respectively, were smartphone owners. Notably, the latter figures are higher than those found in a survey (by the Economist Intelligence Unit – EIU) of healthcare professionals worldwide (91%), suggesting that the UK might be a more fertile ground for digital innovations as means to improving health and social care.
The Imperial College London study provides another interesting insight: 90% of surveyed doctors and 67% of surveyed nurses said they use mHealth apps in everyday clinical practice, to enhance task efficiency and facilitate communication, data sharing and diagnostics.
There are currently around 350,000 mHealth available to patients and health professionals on the Apple and Google Play app stores.
They can gather, share and analyse streams of biometric data such as heart rate, blood pressure, body temperature and physical activity. And, drawing on evidence from medicine and behavioural research, they can facilitate disease prevention by promoting healthy lifestyle behaviours, and assist with the diagnosis, management and monitoring of specific medical conditions. The IMS estimates that the number of iOS apps with these capacities has increased by over 100% since 2013.
In many important areas, health and care systems in Europe and the UK face a growing number of significant challenges. Organisations such as our National Health Service (NHS) are under unprecedented pressure to deliver high-quality services in spite of increasing demand and shrinking budgets.
If new ways of addressing these problems are not found, there could be important consequences for patients and service users. In this regard, the main challenges facing health systems and society fall into six main categories:
| · Population ageing
· Chronic illnesses · Mental health |
· Medication adherence
· Staff shortages · Budget cuts |
Population ageing is defined as the rising average age of a community, and is a global phenomenon. By 2050, the number of people worldwide aged 60 years or older will more than double to two billion, according to the World Health Organization (WHO).
By about the same time, the proportion of the UK’s population over 65 years of age will increase to 24.6%, from 17.8% in 2015, corresponding to an extra 10.8 million baby boomers than in 1975. The Office for National Statistics (ONS), which released the figures in 2017, reports that population ageing will be a key driver of the overall growth of our country, which, within three decades, will have the largest population in Europe.

Increased life expectancy is, with declining fertility rates, the reason why populations age. Indeed, we live longer today than ever before. For example, in 1901 people in England had a life expectancy of 45 to 49 years. This has risen to about 79 to 83 years in 2012, and is projected to further rise to about 83 to 86 years by 2032.
Increased longevity is certainly one of the greatest achievements of the twentieth century. Nevertheless, from a health resources standpoint, it creates significant challenges.
These are mainly in terms of the ability to cope with increasing demand for health and social care, given that, as life expectancy expands, so does the period of time prior to death in which people may experience disease.
Notably, life expectancy is increasing partly because the leading cause of death within older populations has shifted from acute illnesses to chronic conditions.
Over 15 million people in England have at least one chronic disease, such as arthritis, cancer and diabetes. Although diverse, these conditions share one common feature. They have profound, long-term effects on all aspects of life – psychological, social and economic – creating unprecedented challenges for patients and health providers alike, in Britain and worldwide. For example, people with chronic conditions are the most frequent users of health services. In England alone they account for more than 50% of all GP visits and 70% of all hospital bed days.
An important fact about physical chronic conditions is that they are commonly associated with mental health problems such as depression, anxiety and, in older adults, dementia. The latest available figures suggest that every year a quarter of the UK population experience mental health problems, but only about 15% receive adequate care. Yet, mental disorders cost the economy £105 billion annually.
Since two million more people are expected to experience mental issues by 2030, it is clear that the NHS faces huge present and future challenges in this area. Unsurprisingly, experts recommend that creating integrated models of care that enables to address both physical and mental health needs in individuals, should be a main priority for clinical commissioning groups.
Adding to the challenges facing health systems is the large proportion of patients who don’t take their medications. A survey by Omnicell revealed this occurs in 21% of cases, mainly among individuals with chronic conditions. For patients, the health consequences range from poor quality of life due to uncontrolled symptoms to increased risk of complications, hospitalisations and mortality. For the NHS, medication non-adherence means wasting £300 million of medicines every year, according to Government estimates.
A report by the National Audit Office (NAO) shows that in 2014 the NHS had around 50,000 fewer clinical staff than it needed. Another report, by the Health & Social Care Information Centre (HSCIC), found that in September 2015 the NHS had 69,317 fewer staff than previously thought. The situation is expected to further deteriorate as Britain prepares to leave the European Union (EU) following the Brexit vote. Currently 135,000 EU nationals work within the NHS and the adult social care sector. New regulations may affect the ability of European citizens to work in the UK, and prompt part of the EU workforce that is already in the country to move elsewhere.
Currently, over 40% of the NHS budget is spent on people over 65 years of age. An 85-year-old person costs the NHS an average of £7,000 per year – seven times more than the spending for someone in their 30s. Since the population is ageing, these expenditures are expected to increase by up to 1.12% annually. In spite of this, the Government has announced that in the financial year 2018-19 the NHS budget per person will be cut. As the spending per person goes down, the consequences for patients could be serious, as a recent study in the Journal of the Royal Society of Medicine suggests. It found that underfunding in the NHS could be implicated in nearly 30,000 excess deaths that occurred in England and Wales back in 2015.
Although mobile health apps are not intended to replace patient-doctor interactions, it is now widely acknowledged that they have a significant role to play in helping address the multitude of challenges facing health and social care providers, in the UK and globally.
In health and social care, mobile health is driving change in many innovative ways. For example, apps can assist in shifting the focus from expensive, reactive and mainly hospital-based care to care that is less costly, more proactive, and delivered closer to the patient. They can help healthcare professionals work collaboratively with patients and providers for the more effective day-to-day management of chronic physical and mental illnesses.
Overall, mHealth apps have the potential to facilitate the delivery of high-quality care and services to an increased number of patients, within available staff and budget resources.
In a recent interview to the Financial Times, NHS Chief Clinical Information Officer, Keith McNeil, said: “In five years’ time, smartphones … will take the burden away from the limited number of human specialists we have. People will get … triage that’s personalized to them from their phones, or be empowered to look after their own chronic conditions, like diabetes, via home monitoring.”
Collaboration between care providers and the mobile health industry has already started, and the benefits of app usage to healthcare professionals, patients and the public are becoming apparent. The following case studies.
CANTAB MOBILE memory impairment detection app. Developed by Cambridge Cognition Holdings, CANTAB Mobile is a simple, 10-minute, self-guided iPad test that can help identify the first signs of clinically-relevant memory impairment.

It is essentially the mobile version of a test that has been used for over 30 years in clinical trials of Alzheimer’s disease. The idea behind the app is that it can assist healthcare professionals in making more accurate diagnosis of dementia illnesses at a larger scale and at the point of care, so that more patients can receive timely treatment. Clinicians who used the app have seen their rates of diagnosis improve from 39 to 46%. Economic models have estimated that routinely using CANTAB Mobile in GP practices could cut diagnostic costs in dementia by 40% and save the NHS £33 million are just a few examples.
CORDIO voice monitoring app for heart failure. This smartphone app can accurately detect from the tone of the user’s voice whether their congestive heart failure (CHF) is deteriorating. The patient’s voice is captured by the app and sent for analysis to determine the presence of fluid in the lungs – a characteristic symptom of worsening CHF. If fluid build-up is detected, alerts are triggered, so that the patient can receive the necessary treatment, and avoid hospitalisation. For example, they may be recommended to take an additional dose of a heart medicine. In clinical trials, CORDIO has accurately predicted admission to hospital one week before patients’ CHF exacerbated. The voice-monitoring app is now being considered for use among NHS patients.

EPI flu outbreak tracking app. In 2015, England and Wales recorded the largest increase in number of deaths from seasonal flu since 1968. An extra 28,189 people died compared with the year before. Globally, flu outbreaks cause 250,000 to 500,000 deaths annually. A US team of researchers from Duke University and the University of North Carolina at Chapel Hill has developed a smartphone app that can predict how the disease will spread within a community. The app works by collecting real-time data – such as heart rate, blood pressure, social interactions, and physical activity levels – which allow to estimate a person’s risk of developing flu. The information can be used to implement preventive or other measures (e.g., vaccination, throat swabs, improved hand washing) to contain the outbreak and potentially reduce mortality. The team successfully tested the app within a study of university students, the results of which were presented at an International Conference on Knowledge Discovery and Data Mining, in Sydney, Australia.
MY MRI at KING’S app. Physicists at King’s College Hospital, London, created a virtual reality (VR) app that helps reduce the anxiety children often experience before and during a magnetic resonance imaging (MRI) scan. The medical app uses 360 degree videos that can be viewed on a VR headset, smartphone or tablet. Incorporating sound effects and supported by the explanations of a radiographer, the videos allow children to experience an MRI scan before of the actual procedure, both at hospital and home. The app has been successfully trialled by a 10-year-old patient with brain tumour, who needs regular MRI scans. “Even though my dad explained I couldn’t imagine what it would be like … the app is really helpful as it shows you what to expect … it really feels like you are inside the machine,” he said.
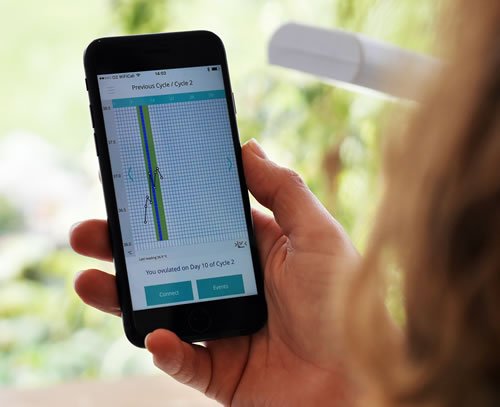
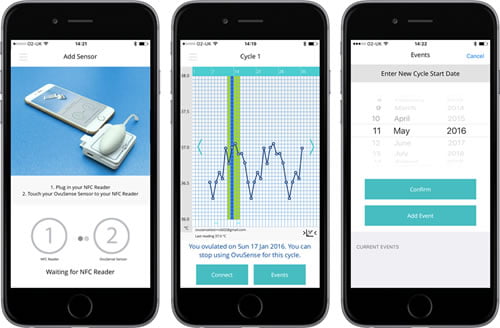
OVUSENSE fertility sensor and app. For couples trying to conceive, the ability to accurately identify the time of ovulation increases significantly the probability of pregnancy. Fertility Focus developed OvuSense, a combined sensor and fertility app, which can predict the date of ovulation up to 24 hours in advance, with an accuracy of 99%. The device consists of a vaginal sensor that collects temperature readings during a woman’s cycle. Using the same technology of contactless payments, the readings are downloaded to an app and displayed on a smartphone or tablet. Unlike other fertility kits, OvuSense is a regulated, CE marked medical device, and is clinically proven. It has enabled couples to conceive, even when pregnancy was deemed unlikely due to the presence of medical conditions such as polycystic ovarian syndrome (PCOS).
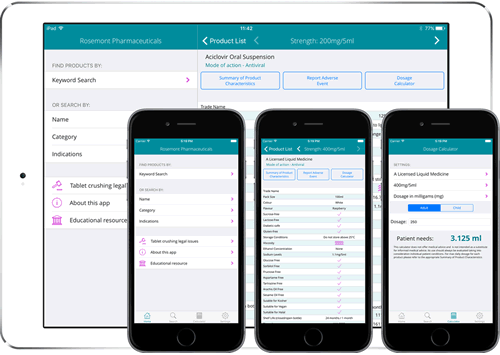
ROSEMONT pocket reference guide dosage calculator. Rosemont Pharmaceuticals Limited have created the first pharmaceutical app to carry the CE Mark and be certified by the Medicines and Healthcare Products Regulatory Agency (MHRA). The app allows healthcare professionals to access information on all Rosemont liquid medicines and easily calculate the accurate dose titration for any adult or paediatric patient. Downloadable on both tablets and smartphones, the app is playing an important role in helping Rosemont Pharmaceuticals successfully engage with customers and, at the same time, contribute to the health and wellbeing of patients by enabling healthcare professionals to prescribe more suitable forms of medication to patients with swallowing difficulties.
The Five Year Forward Review, published in 2016 by the Mental Health Task Force, predicts that mobile health solutions will play a pivotal role in driving positive changes in mental health services. Such transformation is already under way, and clearly encouraged. The NHS offered £400,000 for digital innovations that can help better manage mental disorders and improve access to relevant care and services
Mobile apps have significant potential in this regard. Those that enable the self-management of mental health issues such as anxiety, depression and stress are especially sought-after, given that the majority of affected people avoid seeking face-to-face professional help, largely due to the stigma associated with mental disorders.

Mental health apps can help providers increase access to recognised psychological therapies, so that patients don’t miss out on the benefits. For example, certain smartphone apps offer self-guided versions of cognitive behavioural therapy (CBT) – one of the most effective treatments for a wide range of disorders, including depression, post-traumatic stress disorder and phobias. The effectiveness of these tools is increased if supported by email or SMS reminders, to ensure engagement with therapies. Not surprisingly, patient demand for mental health apps that facilitate self-management is strong. A survey appeared in the Journal of Medical Internet Research found, for example, that over three-quarters of respondents would use mobile apps for managing mental problems, if the tools were available for free.
For mental disorders such as dementia, there are opportunities for mHealth apps to assist care providers in optimising interventions that support independent living and wellbeing. Among various examples is a smartwatch developed by researchers from Gjøvik University College, Norway. The device collects biometrical data such as blood flow and body acceleration and temperature, from which healthcare professionals can extract useful information about the person’s daily activities (e.g., walking, sleeping) and health status. According to data from the Use of Smartwatches for Health Monitoring in Home-Based Dementia Care report, it may be possible to recognise an increase in the number of visits to the bathroom, which may indicate the presence of a urinary infection.
A potential downside of self-management is that patients may lose the motivation to engage with their apps. Gamification provides a means to overcome this potential problem. It involves the use of elements typical of game playing, such as setting goals and scoring points, to enhance the user’s motivation. For example, Public Health England’s Active 10 tallies minutes spent walking and awards badges for reaching daily walking goals established by the user.
In Canada, Vancouver-based Aygo has developed a gamified health app, called Empower, for chronic conditions such as diabetes. Through a game-based approach, patients can set goals for desired changes in behaviour, track medication use and receive reminders for activities. In addition, they are awarded points and badges for any completed task.
The research on the effectiveness of gamified health apps is still limited but promising. For example, a review of 10 clinical trials concluded that game-based app for depression can have a moderate positive effect on symptoms.
Wearables are considered major drivers of mHealth penetration. Although the majority are designed for the wrists, chest and arms, they also include devices that can be embedded in clothing and shoes.
An emerging new trend is wearables for the ear. Like most smart technology devices, they can monitor heart rate and steps taken. In addition, they can provide information about respiratory rate, oxygen saturation and blood pressure in a completely non-invasive way. Some can also calculate caloric intake and provide dietary suggestions. Since wearables like these collect data in a passive manner, they can be particularly useful for older adults, who may not be technology savvy.

More generally, for people with chronic conditions, wearables can monitor symptoms and other parameters in the background, and alert them if changes occur that require medical intervention. An example is the Health and Environmental Tracker (HET) system, developed by researchers of North Carolina State University for people with asthma. It consists of a wristband and a chest patch, which monitor environmental factors such as pollutants and humidity as well as physiological parameters. Complementing the system is a smart spirometer that measures lung function. The gathered data are transmitted wirelessly to a computer, and allow to predict the occurrence of asthma attacks.
Importantly, connectivity among mHealth devices has improved significantly in recent years. For example, wearables can now communicate with health apps on smartphones or tablets. In addition to increasing accuracy and convenience, this opens up a wealth of new opportunities, including the ability to forward health data from wearables to hospital and doctor office systems, so they can be accessed in real-time by healthcare teams for the benefit of their patients.
While there is no doubt about the potential of digital technology as a facilitator of transformation in health and social care, it has its own challenges in terms of mainstream adoption.
A global Economist Intelligence Unit (EIU) survey of doctors and payers, commissioned by PwC, suggests that a major barrier to a greater deployment of mHealth is the technology divide between private and public sector. For example, the survey shows that 33% of public-sector doctors don’t have mobile internet at work, compared with just 14% of those in the private sector. As the survey authors point out, to accelerate the mainstream adoption of mHealth, the public sector must work toward closing this gap, by investing more in technological innovation
Although smartphones and apps are great enablers of the healthcare revolution, they are vulnerable to a number of security threats. These include malicious software that can alter sensitive information or send it to untrusted entities, and third party attacks with theft of private information. Leakage of personal information may also occur, for example as a result of poorly designed apps.

In 2016, the NHS Choices Health Apps Library was suspended following concerns that some of the apps were leaking data. The previous year, a study in BMC Medicine, led by Kit Huckvale of Imperial College, London, had found that 23 of the 35 health apps in the NHS library that were assessed for six months were sending unencrypted identifying information over the internet.
Ideally mobile health devices should be able to seamlessly connect to provider systems, enabling data sharing between, for example, consumer apps and electronic health records (EHR). However, this level of interoperability is presently hard to achieve, largely because of the many disparate platforms and operating systems, which are difficult to integrate with each other. The fact that these evolve at a much faster pace, compared with medical devices, also add to the challenge of speeding up mHealth adoption.
A surprising finding of the survey of hospital doctors and nurses mentioned earlier is that, although the majority used health and medical apps in clinical practice, only a few recommended them to their patients.
A frequently reported reason for this is the inability to assess the quality of the large number of mHealth apps presently available to patients, or the scientific evidence supporting their use. Indeed, the rapid proliferation of mHealth apps seen in recent years, combined with a current lack of agreed quality standards, are making it difficult to distinguish the good from the bad among the multitude of products available. Additionally, a lack of clear regulatory requirements for prescribing health apps could be further contributing to doctors’ reluctance to recommend these products to their patients.
Regulatory initiatives are being undertaken, at both national and international level to bring some clarity. In the UK, the National Information Board (NBI) outlined, in their Personalised Health and Care 2010 policy paper, new endorsement models for consumer-oriented health apps. The initiative, which involves Public Health England (PHE) and the National Institute for
Health and Care Excellence (NICE), among others, aims to ensure that these products meet certain evidence-based criteria in order to be accredited for use. In addition, the Medicines and Healthcare products Regulatory Agency (MHRA) published updated guidance for software developers on health apps as medical devices.
More recently the European Commission has updated the regulatory framework that ensures the safety and efficacy of medical devices to provide some specific guidance relating to medical device software including mobile applications. Come May 2021 all medical device manufacturers will need to follow the MDR and not the MDD.
Over in the United Sates, in 2019 the FDA issued a further update to: “The Policy for Device Software Functions and Mobile Medical Applications Guidance”, first issued in 2013 as “Mobile Medical Applications” (MMA guidance). The FDA updated the guidance to reflect changes to the device definition in accordance with Section 3060 of the 21st Century Cures Act, which created a function-specific definition for device.
In the UK the NHS have developed the NHS Health Apps library, to direct both patients and clinicians to health and well-being apps that they deem are safe to use.
We’re also starting to see specialist companies like ORCHA offer services designed to help healthcare organisations identify health apps that will safely make the biggest impact in terms of improving patient health outcomes.
A characteristic feature of mHealth technology is that it is constantly evolving. Following are the top trends that, according to experts in the field, will shape its future.
In healthcare, as in other industries, digital technologies are generating a huge amount of information, or Big Data, and will continue to do so in the future. These are extremely varied, ranging from clinical data and sensor-generated data to personal health information and electronic medical records. Systems that can support such large volumes of data are expected to increase, and to play a major role in the improvement of health and social care.
For instance, big data health platforms may enable healthcare providers to analyse and compare biometric information or patient behaviour patterns from mHealth apps with the aim of identifying interventions that can help improve care and services, lower costs and make disease prevention more effective.
Emerging smarter technologies of particular interest are those aimed at supporting disease management and adherence to treatment.
A key area in this regard is diabetes. The global devices market for this condition is projected to reach $41.6 billion by 2027. And smarter technologies are increasingly contributing to the market’s growth. An example is the smartphone app Sugar.IQ. It monitors blood glucose levels in real time, and makes food and other recommendations, helping patients keep their diabetes under control.
In the area of medication adherence, coming to the digital health market are smart pills that aim to revolutionise patient compliance with prescribed treatments. For instance, Proteus Digital Health and Otsuka Pharmaceuticals have developed a device consisting of an ingestible sensor linked to a wearable patch that is applied to the chest. Used in combination with a medication, the sensor communicates the intake time to the patch, and the information is then displayed on a mobile app.
In augmented reality (AR), the real-view from a smartphone or tablet device is supplemented with computer-generated elements, such as graphics and sound effects. It is essentially an evolution of virtual reality (VR). Together, these technologies offer fascinating opportunities for the advancement of mHealth.
Some breakthrough platforms are already on the market. Take Medical Realities’ Virtual Surgeon, for instance. Using 360 degree videos and 3D, it enables consultants to practice operations via head-mounted systems such as Oculus Rift, without actually being in the operating theatre. Another example is Bravemind, which uses VR technology to treat people with post-traumatic disorder (PTSD), by having them relive the traumatic events that triggered their PSTD – an approach known as exposure therapy. A similar VR solution has been developed by health tech firm, Pious, for the treatment of people with phobias. And Switzerland-based Mindmaze has created a virtual reality-based intervention for the early motor rehabilitation of stroke survivors.
It is estimated that the VR market alone will be worth around $19 billion by 2020.

A number of medical VR tools have also already been tested for efficacy, in clinical trials. A recent example is the virtual reality intervention for managing pain in hospitals, developed by researchers at Cedar-Sinai Medical Center, in Los Angeles, California.
Using a Gear Oculus headset fitted with a Samsung Galaxy phone, hospitalised patients with pain can engage in a 15-minute, 360-degree, fantasy-world game called Pain RelieVR, which involves shooting balls at various targets. In a six-month study, published in the Journal of Medical Internet Research Mental Health, RelieVR reduced pain scores by 24% – almost two times the decrease observed in a control group of patients with pain who watched a relaxing nature video on a TV screen.
Today, implantable devices are limited to such things as pacemakers or defibrillators. But the future of mHealth includes sensors that will be implanted under the skin and, similarly to what wearable devices do, will gather biometric and other data. Among the various possible applications of these sensors is to assist with wellness, medication adherence and disease management. Researchers at Texas A&M University are developing an implantable sensor that can provide continuous blood glucose measuring, improving the way diabetic patients can control their condition and reducing their risk of complications.
Meanwhile, the National Institute of Biomedical Imaging and Bioengineering (NIBIB) launched the Bionic Man – an interactive tool that details 14 sensor-based and other technologies that may, one day, help prevent, cure or manage injuries and disease. Among these are blood glucose sensing contact lenses, a microneedle patch that delivers vaccines, robotic leg prosthesis, implantable sensors for prosthesis control, and a wireless brain-computer interface that could help people with paralysis move robotic limbs with their thoughts.
COVID-19 has had a massive impact on driving forward the development of mHealth technologies and apps to help track and trace virus outbreaks.
Smartphone apps been widely used to help limit the spread of coronavirus. A number of of countries are now developing their own apps to trace the virus’ journey.
The idea is that such digital contact tracing will identify people potentially exposed to the coronavirus who should self-isolate – thus helping to prevent the virus from spreading further and faster.

Apple and Google also teamed up to develop technology which uses Bluetooth to detect when someone who downloaded the app has spent time near another app user who later tests positive for the virus. Technically speaking what they’ve built is not an app, it’s an Application Programming Interface (API), which is a software intermediary that allows two applications to talk to each other. The idea is that public health authorities will incorporate the API into their own apps that people install. According to Apple and Google their technology is designed to make existing track and trace apps work better.
Developing these types of contact tracing apps is not new. During the 2014–2016 Ebola outbreak in West Africa, contact tracing was a core surveillance activity. However, the health authorities faced some significant challenges with paper-based contact tracing systems that needed addressing. These include incomplete identification of contacts, delays in communication and response, loss of contact lists, inadequate data collection and transcription errors.
A proof-of-concept study was commissioned on: The Use of a mobile application for Ebola contact tracing and monitoring in northern Sierra Leone. The aim of this study was to design and evaluate an electronic system for tracing contacts of Ebola cases. The system featured data capture using a smartphone application, linked to an alert system to notify the District Ebola Response Centre of symptomatic contacts.
The World Health Organisation (WHO) has also developed an electronic tool called Go.Data. The mobile application is available for Android and iOS and is designed as a flexible tool for field data collection, focusing on case and contact data including contact follow-up.
In recent years, mHealth has evolved significantly. Users increasingly rely on a multitude of technologies to better manage physical and mental conditions, prevent disease and share information. There is an increased opportunity for care providers to improve health and social care, by empowering patients, healthcare professionals and the public, and by investing more in mHealth technology innovations.

As discussed in part 1 health and medical apps are beginning to have a profound impact in terms of new methods of delivering healthcare and new techniques for managing health and well-being
In this section we’re going to talk specifically about the app development process and what you’re going to need to consider right from the start in-order to develop a successful and fully complaint app.
Rushing headlong into the development phase without first having a strategy and plan in place very rarely leads to a positive return-on-investment when creating any form of mobile app.
Whether your goals are about improving clinical interaction between HCPs and patients, health and fitness measurement, medical education or hospital administration, adopting a strategic approach to your app project is more likely to deliver maximum commercial impact.
Think about how your app can help to develop a stronger healthcare system.
mHealth strategy considerations:
Engage with patients & HCPS
One of the most important things you can do is to engage patients and healthcare professionals as earlier as possible during the solution design phase. This will help you to identify key design features and functionality that will match the goals of your end-users and help you to develop an app that will have a high engagement rate. According to ORCHA, an organisation which reviews and evaluates health apps, just 43 apps are responsible for more than 83% of all health app downloads, and that over 80% of health apps don’t get more than 5,000 downloads. These numbers confirm that the health apps market is still in its infancy, with much experimentation going on, and a surprisingly small proportion of apps achieving success.
Competitor Research
Carrying out research on your potential competitors is a vital part of the new product development process. This will help you to assess if your app will be unique or if something similar already exists on the app stores. You can use sites like iMedical Apps, MobileHealth News and the NHS Health Apps Library to do some initial research and of course there are the medical and health and fitness categories on the Google Play and Apple Store app libraries.
Once you have developed your health app strategy and produced a technical and functional specification for your app you will typically follow this development process.
UI/UX Design & Wireframing
This involves producing storyboards which help to visually demonstrate the user journey and the app’s key features like navigation and showing where functionality, such as buttons and other tools are required.
The next step involves producing a mock up version of the finished design – where the wireframe is your blueprint showing the structure of each page, the user interface design allows you to see how the finished pages will look on a particular device.
App Prototyping
If you are in the early stages of creating your app and need to test the concept first or obtain funding then you should consider investing in a prototype of your app. Prototyping is a cost-effective way of visually showcasing your idea to stakeholders or potentially investors as it does not involve building a fully functioning app that has been coded. A prototype will help you to more easily demonstrate the key design features and functionality of your app idea.
Engineering & Programming
During this phase programmers begin coding the app for the various platforms and devices. This typically involves developing apps for Android smartphones & tablets, iOS devices including the Apple Watch but it could also involve developing an Alexa or Google Home skill.
Any integration with software systems (such as APIs, databases, patient information systems, electronic health records – EHRs etc.) are also implemented by the development team during this stage. At this stage your developers would also implement any connectivity solution that needs to take place with a smart medical device.
If your app collects and stores any sensitive medical and patient data then you will also need to ensure that the data is being stored securely. The “Big 3”: Google, Amazon and Microsoft offer cloud-based storage systems specifically for storing medical data.
If you’re developing a medical device app then you will also be required to produce a Technical File for CE marking purposes so that your app complies with the regulations set by the Competent Authority for each country your app will be marketed. So, the FDA for North Americana and the MDR for UK and Europe.
Testing, Compliance & Deployment
The final stages in producing a fully functioning app involve user testing, data security testing, compliance checking (including applying the CE Mark to your app if it is classed as medical device software – MDSW), installing app analytics and creating all the app store marketing assets.
Only once all these stages have been completed and signed-off, should you submit your app to the app stores to avoid app store rejection.
Medical compliance and data protection needs to be considered when developing medical mobile applications that will be in the public domain. The MHRA in the UK, the EU and the US all have specific guidelines which need to be adhered to when developing apps that meet the definition of a medical device or medical device software (MDSW).
The first stage is to assess whether your app product meets the definition of a medical device and what class it falls under, i.e. Class I, Class II a, Class III etc. If it does then it will need to conform with the medical device regulations (MDR) for Europe and section 510(k) of the Food, Drug and Cosmetic Act from the Food and Drug Administration (FDA) for North America.
Your in-house medial compliance officer or external partner will need to assess the need for CE marking your app. If it does require CE marking then you will need to develop the appropriate quality management system within your business; follow the correct software development processes and adhere to the relevant harmonised standards so you can produce the necessary paperwork for your Technical File. Meeting the requirements of the appropriate regulatory frameworks and applying CE marking has to take place before the medical device app can be placed on the market. Failing to do could result in your app being rejected from the app stores.
If your app connects to an existing medical device using Bluetooth and/or NFC technology then your app will become part of the overall medical device product and follows the same class as the hardware device.
The main ISO quality management systems and harmonised standards that are most likely to be relevant to your business if you are developing a health or medical app are:
Other regulatory frameworks that you should also learn more about would be:
The General Data Protection Regulation (GDPR) and the Healthcare Insurance Portability and Accountability Act (HIPAA) are the legal frameworks for processing health data. As a company, you are either a Data Processor or Data Controller (GDPR), or Business Associate (HIPAA). And as of 2020, you may also have to comply with the new Medical Device Regulation (MDR) if your app meets the definition of medical device software (MDSW).
According to ClearDATA Chief Privacy and Security Officer and Founder Chris Bowen, someone’s medical record is 50 times more valuable than their credit card number.
mHealth apps rely completely on the trust of the end users. Recent studies showed that 59% of people don’t like sharing health data online. Every time there is a data breach, public trust is eroded further. The controversy surrounding the DeepMind Streams app and Google highlights how important maintaining trust is.
As the developer of the mobile application you will be legally responsible for the safe management and storage of all the health data your mobile app collects. This means you must protect your users’ data by implementing recognised data security techniques like pseudonymisation and encryption.
App marketing involves creating digital assets that will you to promote your app and maximise installs.
Just like websites need to be actively promoted to help drive visitors to them, your mobile app needs to be supported by a well thought out app marketing plan if you want people to find and install it.
All app publishers will start with a basic app store submission but you should also consider a specific app store optimisation (ASO) service to help increase the visibility and ranking of your app in the app store search results.
Around 60% of apps are found through app store search, so if your app does not rank high up in the search results for specific keyword searches then chances are your app will struggle to get discovered.
Typical app marketing activities include:
As part of your app marketing plan you’ll also need to consider how you’re going to measure performance. There are a number of app analytics tools that can provide you with data and insights into how your app is performing and how it compares to other apps within your sector. Popular tools include:
Developing mobile applications that function as medical devices is complex. Choosing the right partner is more than just about assessing their technical capability.
Medical app developers also need to understand the regulatory and security requirements. Working with an inexperienced company can prove extremely costly. This could be in the form of a fine due to a regulatory violation but it could also result in app store rejection, meaning that you won’t be able to distribute your app for download.
Search our app developer directory to find a developer that has a deep understanding of the complexities of medical device compliance in mobile application development as well as the intricacies of balancing usability with security and privacy.